Stress Related Proteins
Determination of the structures of stress related proteins
Cells – from bacteria to humans – are in a constant battle with their environment. Attack from other organisms, by chemical toxins, or by outside physical or chemical influences (such as temperature, pH, pressure, organic solvents, etc.) must be counteracted by the cell to survive. Some of these outside influences are counteracted by special stress-related proteins. Some are called heat-shock proteins (HSP), as the amount of these proteins increases when cells are heated beyond their optimal growth temperature. HSPs prevent other proteins to mis-fold, unfold or aggregate under stress.
Some photosynthetic organisms have special proteins that counteract high-light (that can destroy the photosynthetic system) or lack of nutrients. We have determined the three-dimensional structures of HSPs from M. tuberculosis and T. vulcanus, that have very different structures and modes of activity. Other stress related proteins that we have studied are the NblA protein that disassembles the Phycobilisome antenna protein under nutrient stress and the Orange carotenoid Protein system, that protects some cyanobacteria from high-light exposure. We use many biophysical tools to study these proteins function, coupling the results to the structures we determine.
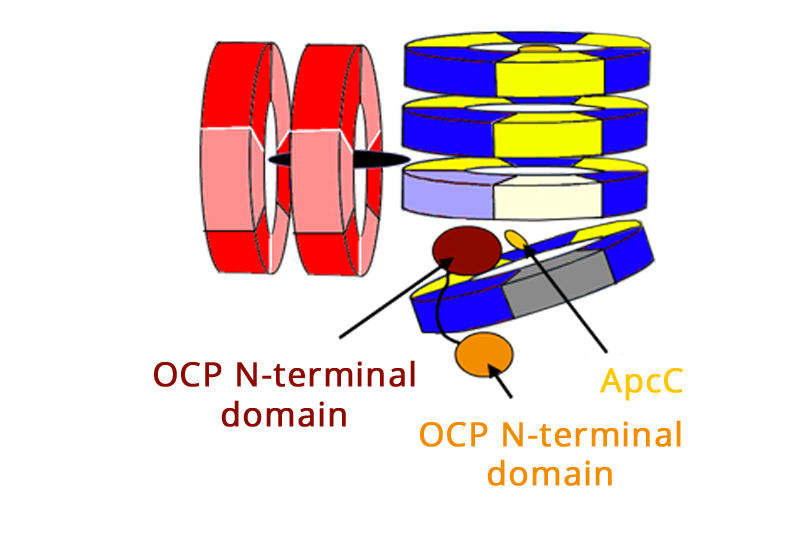
Model of the interaction between the OCP and Phycobilisome preventing damage by high light (Harris et al. PNAS 2016)
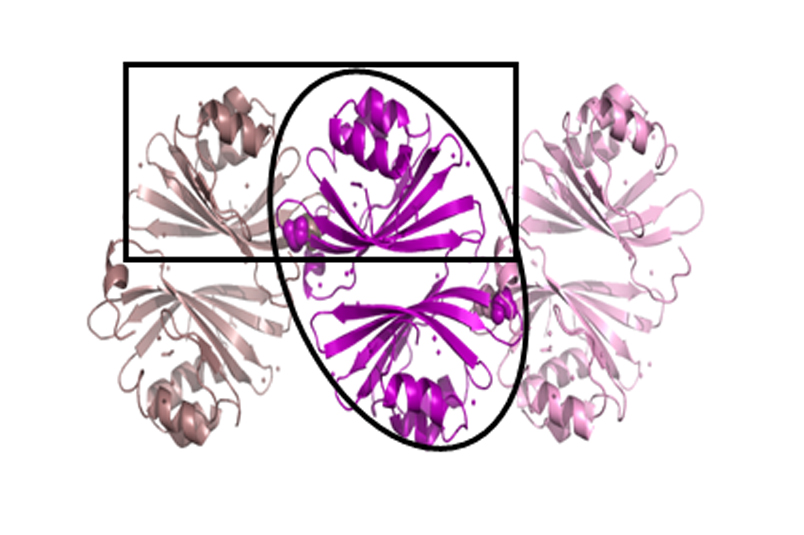
The CTDH protein structure from the OCP system showing to different functional dimeric interfaces

