INTRODUCING
Shira Bar-Zvi
email: shirabarzvi@gmail.com
Structure-Function Analysis of the Phycobilisome Complex of the Cyanobacterium A. Marina
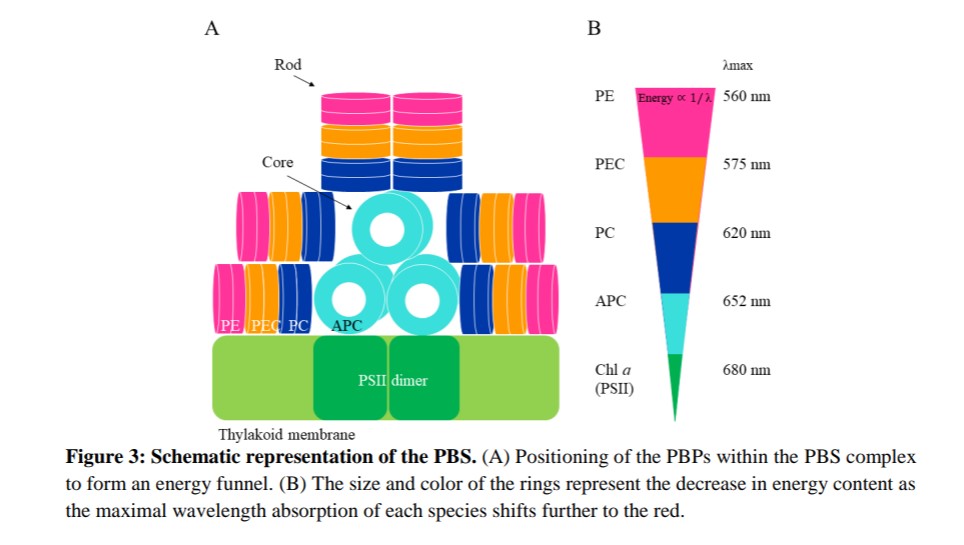
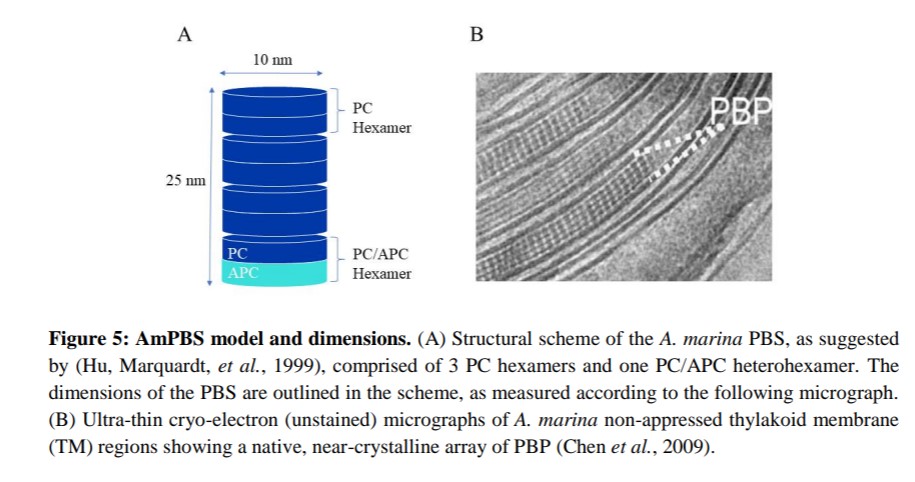
The major light harvesting antenna in cyanobacteria and red algae is the phycobilisome (PBS). The PBS typically contains different subunits, and energy efficiently flows from high (phycocyanin, PC) to low (allophycocyanin, APC) energy absorbing subunits. The smallest PBS identified to date is that of Acaryochloris marina (A. marina), composed of a single rod.
We have determined the crystal structure of A. marina PC (AmPC), the major phycobiliprotein (PBP) component of the A. marina PBS (AmPBS) to 2.1 Å. The basic unit of the AmPC is a heterodimer of two related subunits (α and β); two such α and two β isoforms, the products of simultaneous expression of different genes, turned out to be superimposed in the asymmetric unit of the PC monomer structure.
We have isolated several PC types from the A. marina, of different spectral properties, corresponding with a different PC α and β isoform ratio. These include a PC type exhibiting APC-like characteristics, suggesting it can efficiently serve as the AmPBS terminal emitter. This is the first PC found to date, transferring energy in a way that challenges the currently known PBP classification system.
The lack of APC in the AmPBS, as determined in this study, together with the novel AmPC types, suggest that the AmPBS may be the first PBS to operate without APC, solely based on one multi-functional PC.
Structural analysis of the two PC isoforms protein structure has indicated possible modifications to the phycocyanobilin chromophore protein environment, which may be responsible for this altered energy transfer capabilities of the different AmPC types.
Additionally, combined spectroscopic and kinetic analysis of time-resolved fluorescence and absorption data of this antenna allowed us to identify spectrally different forms of chromophores and to propose a new simplified model of how they may be distributed within the AmPBS structure.