
Coupling photosystem II with Nano-Photocatalysts for overall Water Splitting
Photosynthesis is one of the most primary biological processes on earth, giving rise to all advanced life-forms as they are known today. The full potential to utilize the photosynthetic process for the benefit of humanity has yet to be discovered. Modern nano-chemistry allows the production of a wide variety of nanoparticles with tunable surface chemistry, electrical and optical features, opening new possibilities for the design of synthetic- biological hybrid systems. The biohybrid approach can be particularly useful when it comes to enzymes- biological catalytic unites that significantly reduce the activation energy of a wide range of chemical reactions, produce little to no hazardous by-products, and consume little resources. Photosystem II (PSII) is a powerful biological catalyst, having the ability to absorb the light of the sun and use the energy to oxidize water, which is the essential initiation source for photosynthetic electron transfer. In this research, we have tried to approach the problem from a nano-chemistry direction, forming two main types of models: conjugation of PSII with plasmonic nanoparticles and semiconductor nanocrystals compatible for conjugation with PSII. Each system was tailored according to the specific chemistry of PSII and the goals we wished to achieve. Our lab has previously proven the capability to produce photocurrents using photosynthetic membranes. We, therefore, took it one step forward to produce photocurrent from isolated PSII. Once accomplished, the next step was to design and synthesize compatible plasmonic nanocrystals, well-studied systems in many contexts, including conjugation to enzymes to enhance their activity.
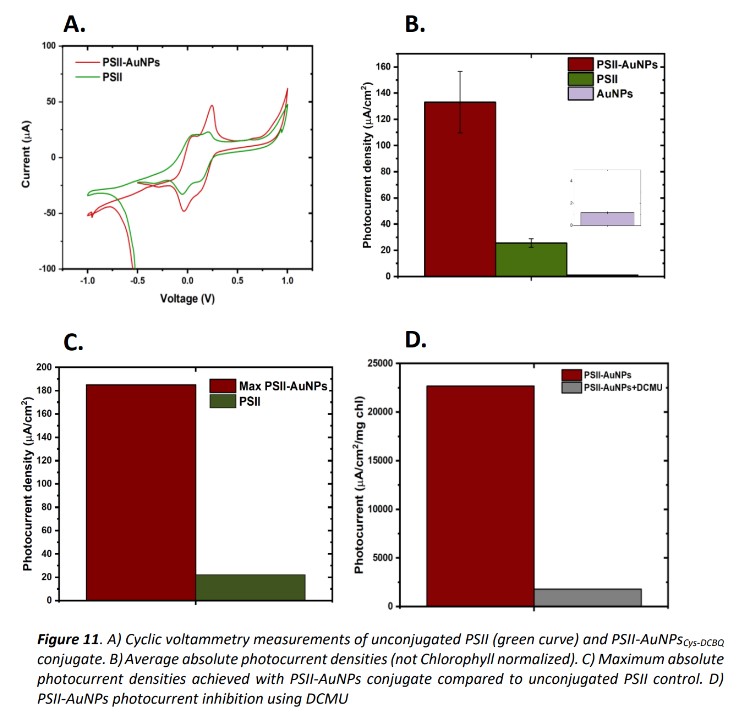
We used cysteamine-capped gold nanoparticles (AuNPs) designed to have an SPR peak at 500-550 nm, matching the gap in chl a absorption (400-450nm, 600-700 nm) and reacted with DCBQ, a known PSII electron acceptor. The conjugation resulted in up to an order of magnitude photocurrent enhancement, compared to unconjugated PSII, and was particularly noticeable in the SPR peak region. For the second project of designing a hydrogen-evolving system that could be conjugated with PSII, we collaborated with the lab of Prof. Lilac Amirav from our Faculty, a leading group in the study of semiconductor nanocrystals and their properties. We chose rod-shaped nanocrystals made of a Cadmium Selenide core and Cadmium Sulfur shell (CdSe@CdS NRs) known to catalyze hydrogen evolution reactions. Our efforts to design NRs compatible for bio-conjugation with PSII gave birth to a unique CdSe@CdS NRs synthesis procedure, providing us with the ability to control the shell thickness, a parameter up until now was almost impossible to control through synthesis. We modified the CdSe quantum dots (QDs) synthesis, enabling different rates of shell growth. The modified QDs enabled the synthesis of three new types of NRs with different shell thicknesses (3, 2, and 1 layers of shell). Characterization results of the new NRs indicated an enhanced charge separation and catalytic efficiency of the intermediate thickness NRs compared to the thickest and thinnest ones. Importantly, and unlike standard CdSe@CdS NRs, the intermediate-thickness rods reached an impressive 35% hydrogen evolution efficiency at neutral conditions in a buffer solution, a crucial property for conjugation with proteins.
Congratulations to Hagit Shoyhat on successful completion of the M.Sc. Degree!
We are proud of you!!!


