Enzyme structure and function
Thousands of enzymes are the chemists of all cells, allowing life to exist.
The special proteins that contain the chemical structures that allow for enzymatic activities transform simple molecules into the complicated macromolecules such as DNA, proteins, polysugars, lipids, etc.
Enzymes catalyze the synthesis and degradation of molecules, perform metabolic and energetic tasks and protect our cells from both internal and external dangers.
Understanding the precise mechanisms of enzyme catalysis is almost impossible with knowledge of their three-dimensional structures.
We are interested in all aspects of enzymes function:
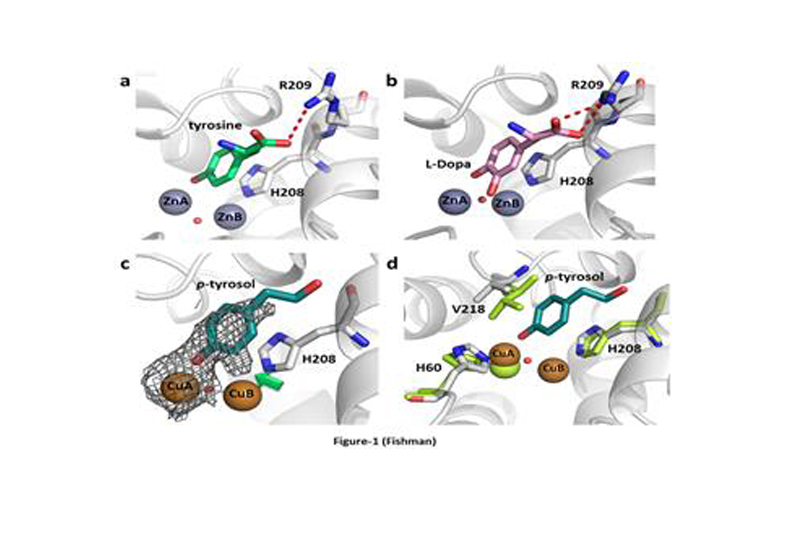
In collaboration with Prof. Ayelet Fishman of the Faculty of Biotechnology and Food Engineering of Technion, we have explored this enzyme, isolated in Ayelet’s lab from a soil bacterium called B. megaterium.
We have solved this enzyme in its active form, showing exactly how it’s substrates bind, how it is inhibited by compounds used in cosmetics, how critical copper ions are uptaken and much more.
- How to they fold and assemble?
- How do they perform their catalytic function?
- How do they bind substrates, inhibitors, analogs, co-factors and solvent molecules?
- What happens if certain amino acids are changed (mutations)?
- How did they evolve from more primitive proteins?
In collaboration with Prof. Ayelet Fishman of the Faculty of Biotechnology and Food Engineering of Technion, we have explored this enzyme, isolated in Ayelet’s lab from a soil bacterium called B. megaterium.
We have solved this enzyme in its active form, showing exactly how it’s substrates bind, how it is inhibited by compounds used in cosmetics, how critical copper ions are uptaken and much more.
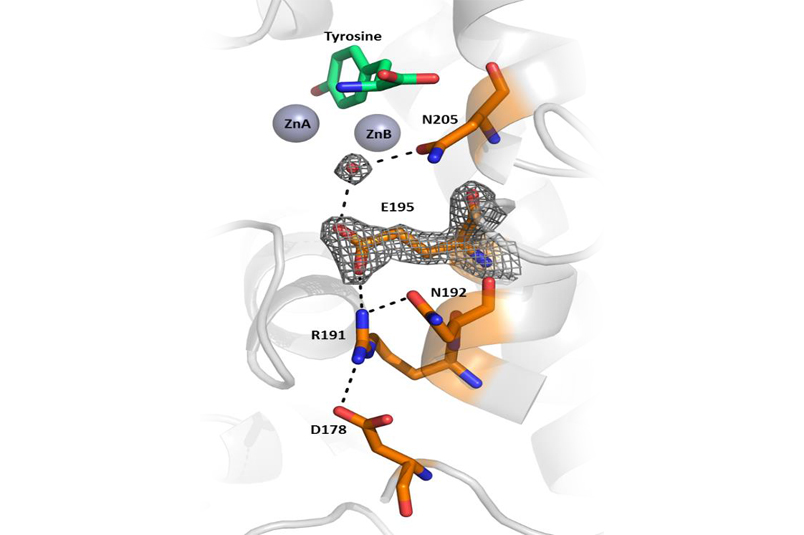
A small portion of the B. megaterium tyrosinase structure showing critical amino acid residues and a water molecule that initiates catalysis by deprotonation of tyrosine
Enzyme structure and function
Enzyme Structure and Function
Publication 1
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.
Publication 2
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.
Publication 3
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.
Publication 4
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.
Publication 5
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.
Publication 6
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.
Publication 7
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.
Publication 8
A detailed answer to provide information about your business, build trust with potential clients, and help convince the visitor that you are a good fit for them.

