
Structure-Function Investigations of the MntA and MntC Proteins from Mesophilic and Thermophilic Cyanobacteria
Manganese is accumulated in high concentrations in various eukaryotic organelles as well as in the cytoplasm of prokaryotes. In the cyanobacterium Synechocystis sp. PCC 6803, the high affinity import of Mn in to the cytoplasm is carried out by an ABC transporter.
This ABC transporter complex contains two transmembrane domains (MntB), two nucleotide binding domains (NBDs) exposed to the cytoplasm (MntA) and a periplasmic, substrate binding lipoprotein (MntC).
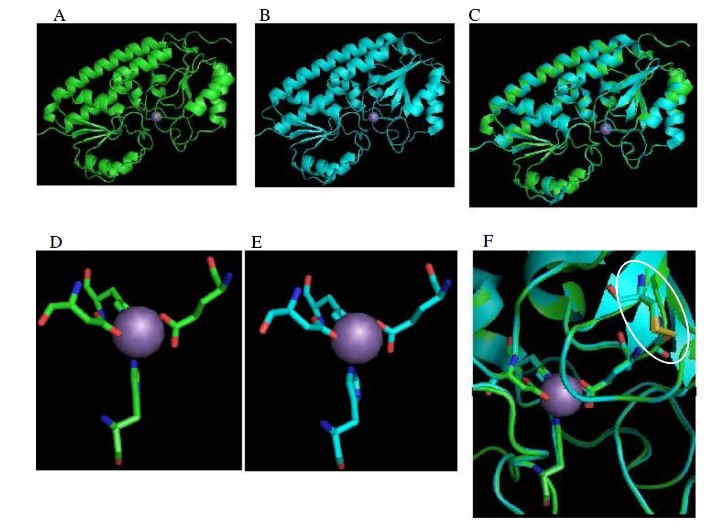
The structure of the MntC protein has been determined using X-ray crystallography to a resolution of 2.9Ǻ. This structure showed the presence of a disulfide bond near the Mn2+ binding site. However, due to the low resolution of the structure, further investigation is required. The most important issues are the exact role of the disulfide bond, that is a specific characteristic of cyanobacterial manganese solute binding proteins, and the specificity of the protein towards Mn verses other metal atoms of similar characteristics.
We started to investigate the homologous manganese solute binding protein from the thermophilic cyanobacterium Thermosynechococcus vulcanus. The rational for this was that perhaps the protein from a thermophilic organism would yield a more stable protein and allow for the improvement of the resolution of the MntC protein structure.
The mntC gene from Thermosynechococcus vulcanus was cloned and large amounts of highly purified protein were obtained. Indeed crystals obtained from this protein diffracted to intermediate resolution, but the structure was unsolvable due to the presence of a large number of monomers in the asymmetric unit.
Functionally important residues are highly conserved among the NBDs, suggesting that the ABC transporters share a common mechanism of coupling ATP hydrolysis to substrate transport. In order for us to understand this mechanism and transport, we need to do extensive biochemical analysis together with the structure determination of the entire MntABC transporter.
We cloned and overexpressed the MntA protein from Synechocystis sp. PCC 6803. We have found that the protein is expressed in inclusion bodies that can be solubilized in 8M urea. We managed to find the best conditions to resolubilize the protein and to prove that the protein has been refolded correctly making it amenable for use in crystallization condition screening but crystals obtained from this protein have not yet yielded X-ray diffraction.
Read MS.c Thesis
Read Ph.D Thesis
Avital's Linkedin Profile


