
Structural and functional characterization of cyanobacterial phycobilisomes
Photosynthesis is the biological process of converting light energy into chemical energy. Organisms such as plants, algae and cyanobacteria, utilize this process to sustain life and carry out many cellular processes. Cyanobacterial cells contain unique membranes, housing the photosynthetic system. The system is composed of many protein complexes, where the two key components initiating the process are antenna complexes and reactions centers (RC). The antenna complexes are called light harvesting complexes (LHC), corresponding to their function.
Upon light irradiation, light energy is absorbed by the LHCs and immediately transferred to a RC, where the chemical and electron transfer reactions occur. The energy transfer (EET) from the LHC to the RC is highly efficient and relies on an overlap between the energy emitted by the LHC to that absorbed by the RC. The current mechanisms do not fully explain the EET occurring inside and between the complexes.
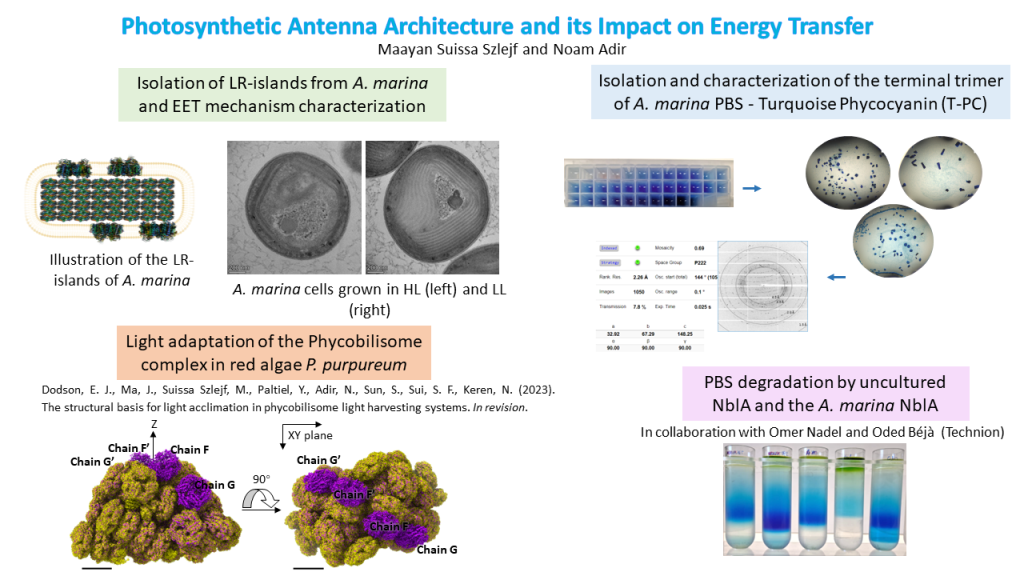
In this research, we investigate this energy transfer in a unique cyanobacteria, A. marina, for which there is a large energetic and physical gap between LHC and RC, yet it remains efficient. In practice, we utilize a unique phenomenon found only in the A. marina membranes, where there is heterogenous separation between domains that contain the main LHC (the Phycobilisome) and domains lacking this complex. These unique domains, that we call LR-islands, contain all the components of the photosynthetic system, which poses an opportunity to investigate the system in its entirety.
We have found that in different light intensities, the organism changes the composition of the thylakoid membranes. In low light there is an increase in A. marina PBS (AmPBS) production and the complex is present in all the regions, as opposed to high light where the unique LR-islands appear. In addition, we investigate particularly the AmPBS complex, which is known to contain only phycocyanin (PC), assembled from two isoforms of the α and β subunits, in varying ratios. Using structural tools such as crystallography and cryogenic electron microscopy (CryoEM), in addition to spectroscopic and analytical tools, we can shed more light on the role of each isoform within the phycobilisome.
Additionally, in collaboration with Sui group (Tsinghua university) and Keren and Paltiel groups (HUJI), we have studied effects of light intensity on the PBS complex from red algae P. purpureum. Following a comparison between low light structure of the PBS (published in 2020) and medium light structure of the PBS (this work), we have found major differences in the subunit composition, as well as the pigments’ locations and quantity. This research elucidates the ability of fast acclimation by an organism which resides in a changing environment and may help in understanding other acclimation mechanisms.
Read MSc Thesis
Who’s Your Neighbor?
Suggesting alternative assemblies of E/F type bilin lyases from crystal lattice analysis
Maayan's Linkedin Profile


