Authors:
Adir, N., Bar-Zvi, S., & Harris, D.
Received 20 April 2019, Revised 19 June 2019, Accepted 9 July 2019, Available online 12 July 2019, Version of Record 25 February 2020.
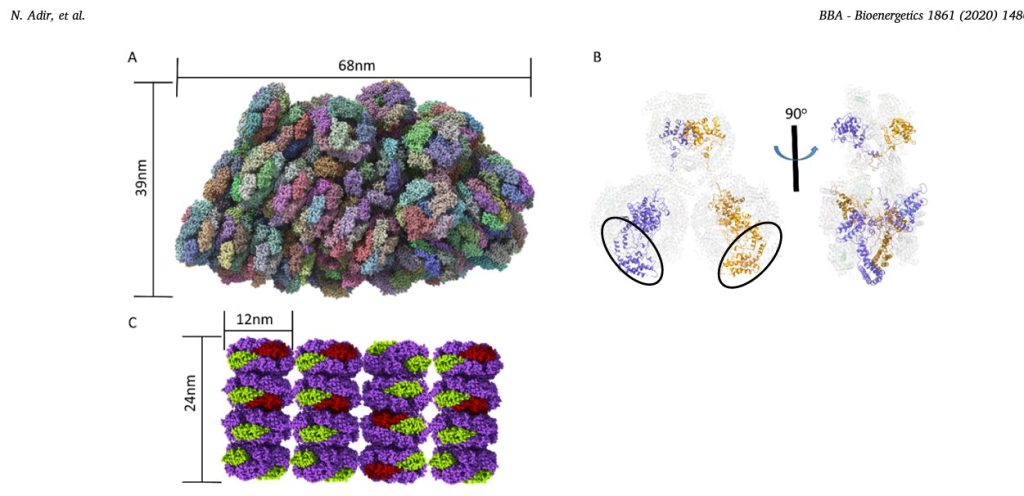
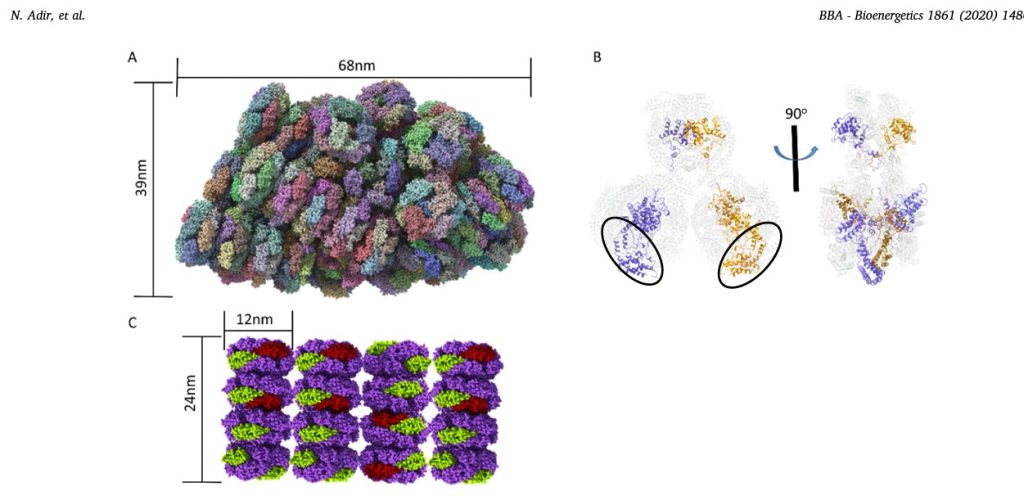
Fig.1. New PBS structures.
A. The structure of entire 16.8 MDa PBS complex from G. pacifica (PDB code 5Y6P,[45]) determined by cryo-EM. Each color represents a different subunit (not all subunits are visible). The membrane bound reaction centers would be found directly below the PBS. The third dimension of the PBS (into the plane of the paper) is 45nm.
B. Cartoon representation of the structure of the ApcE (LCM) linker protein in the G.pacifica PBS core. The APC proteins (in transparent cartoon representation) form three cylinders, two on the bottom (each containing three trimers) and one on the top (with two APC trimers). The two copies of ApcE (in purple and brown) contain a ApcA-like phycobiliprotein domain (black ovals), followed by three linking domains that wind through the bottom cylinders up to the top cylinder. The panel on the right is rotated counter clockwise by 90°, showing how the ApcE juts out of the top cylinder.
C. Model of aligned A. marina PBS. The AmPBS is composed of four hexamers containing PC, in a single rod (1.2MDa), with multiple rods aligned in parallel fashion between thylakoid membranes [61]. The structure of A.marina PC was determined by X-ray crystallography (PDB code 5OOK, [64]) showing that the PC subunits in the AmPBS are from two isomers of both α and β subunits. Here we schematically show the minor α and β subunits in green and red, respectively. The major subunits are in purple. The actual positions of the minor subunits are not yet known and are shown here for illustration purposed only. Molecular graphics were created using ChimeraX. (http://www.rbvi.ucsf.edu)