Authors:
Mor Sendovski, Margarita Kanteev, Vered Shuster Ben-Yosef, Noam Adir, Ayelet Fishman
Journal of Molecular Biology 405, 227-237 (2011)
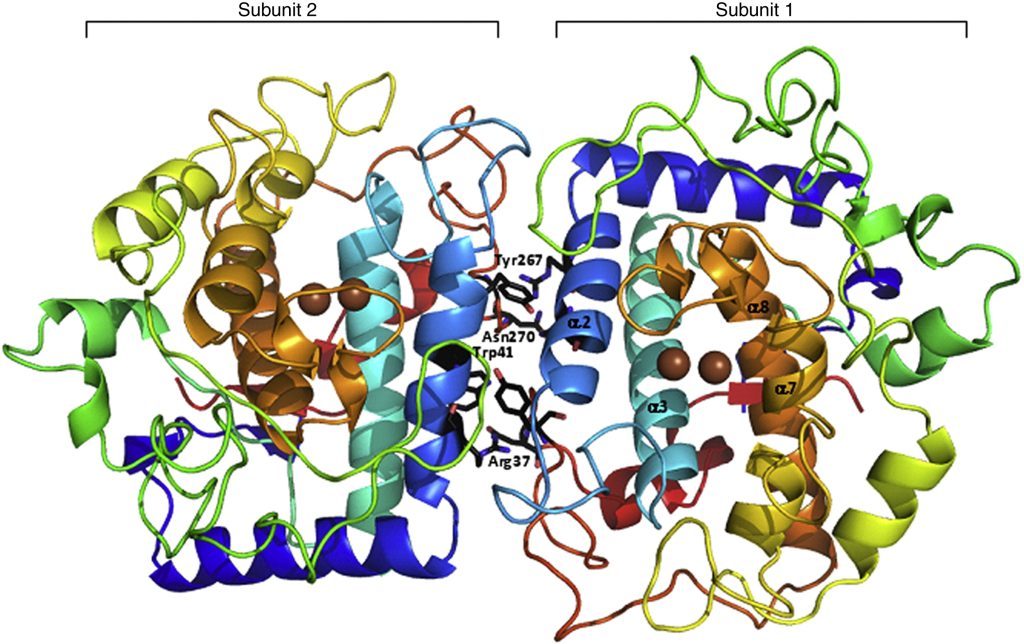
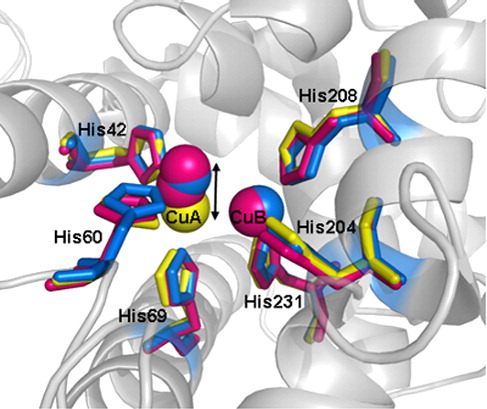
Fig. 1. Overview of TyrBm structure. The dimer structure of TyrBm is presented; both subunits (subunit 1 on the right and subunit 2 on the left) are presented in an N-terminal to C-terminal spectrum. Copper ions in the active site are presented in brown. The dimer-forming contacts in each subunit, Trp41-Tyr267 and Arg37-Asn270, are shown in black. The four main helices, from which the His residues coordinating the copper ions project, are presented in subunit 1: α2 in light blue, α3 in cyan, α7 in dark yellow, and α8 in orange. The structure presented is that of TyrBm1 (a type 1 crystal) crystallized in the presence of Zn ions. The overall structures of all other crystal structures described here are nearly identical. All figures presented in this work were generated using PyMOL (http://www.pymol.org/)
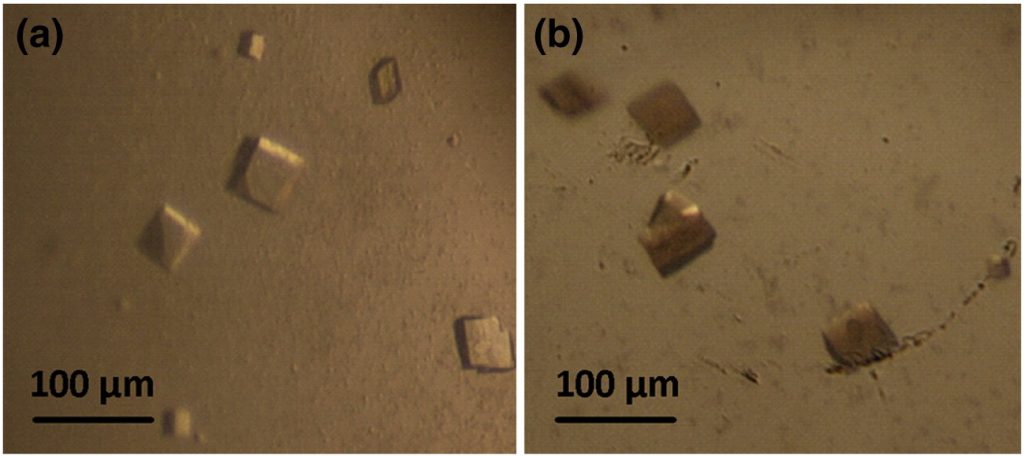
Fig. 2. TyrBm crystals have monooxygenase activity. (a) TyrBm monoclinic (type 2) crystals obtained in drops containing 18% polyethylene glycol 8000 and 0.1 M cacodylic acid (pH 5.6) at 20 °C after 14–21 days. (b) Crystals were soaked in 0.5 mM l-tyrosine and turned brown after 48 h.